Teams arrive to check major vaccine producer


Latest:
Cui Honghai, former head of Jilin drug administration, was prosecuted over bribery in Changchun on Thursday.
In a filing to Changchun City Intermediate People's Court, Changchun City People's Procuratorate said Cui made use of his position to help others to gain benefits and illegally accepted properties from others, thus he should be held accountable.
Previous report
Teams arrive to check major vaccine producer
Progress to be revealed in timely manner, may help reform system
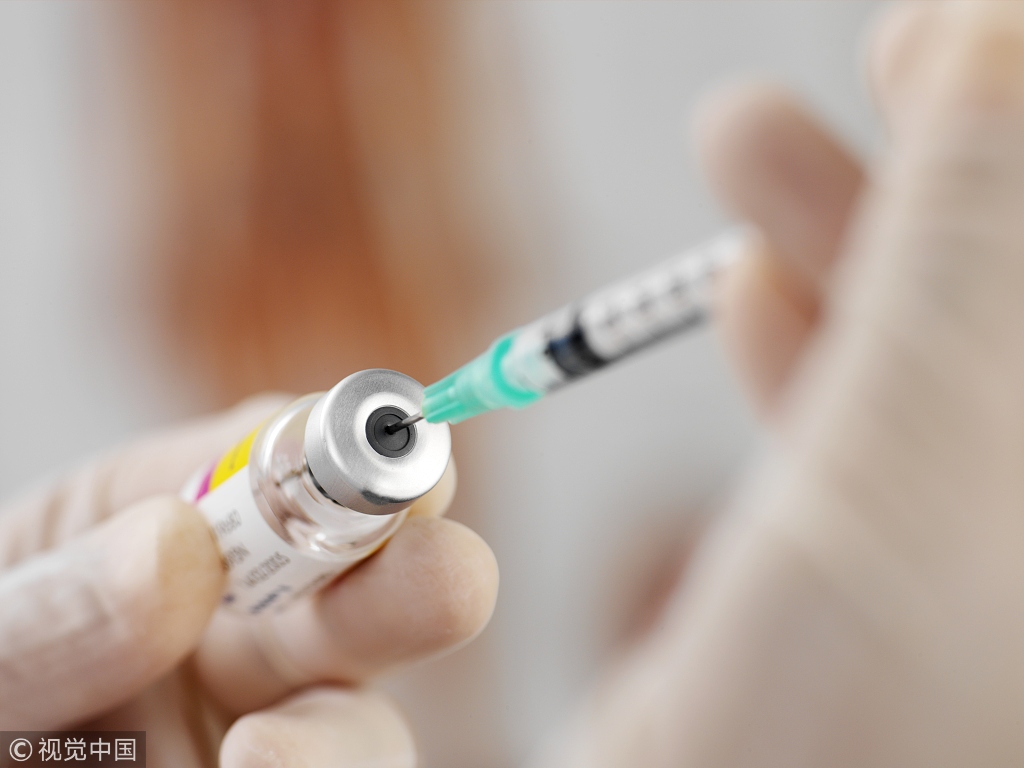
An investigative team dispatched by the State Council, China's Cabinet, has arrived in Jilin province to investigate possible violations of the law involving a major vaccine producer that falsified records about its production of rabies vaccine.
An inspection by China's State Drug Administration on July 15 found that Changchun Changsheng Bio-tech Co, in Changchun, Jilin province, faked production and inspection records.
The incident aroused public outrage in China because of the virtual 100 percent mortality rate of rabies.
Bi Jingquan, Party chief of the State Administration for Market Regulation and head of the investigation team, vowed on Tuesday to pursue a thorough investigation and severely punish all responsible, including government officials.
He said the progress of the investigation will be revealed to the public in a timely manner, and authorities will research how to reform and improve the existing vaccine management system to establish one that guarantees the safety and effectiveness of vaccines.
In a statement released on Wednesday, the World Health Organization said it supports the action of China's State Drug Administration in holding the problematic batches of rabies vaccine to ensure they are not placed on the market.
All of the company's rabies vaccines have been recalled, and no quality problems have been found in vaccines that have already entered the market, the administration said.
"Regulatory oversight of vaccines is critically important. It is the government's primary method of ensuring that the vaccines produced and used in China are safe, of good quality and effective," said Gauden Galea, the WHO Representative for China. "This incident shows that when regulatory oversight works well, potential risks can be averted."
While the current incident is clearly regrettable, the detection of this event by an unannounced inspection shows that the regulatory authority's system of checks and balances to protect the health of the population is working, the WHO said.
Changchun police said on Tuesday that 15 people suspected of criminal violations had been detained, including Gao Junfang, the company's chairwoman.
"The WHO awaits the results of further investigations and stands ready to provide support to national health authorities," the WHO China Office said in the statement.
In a statement released on Wednesday, the General Administration of Customs said no health centers for international travelers had purchased or used rabies vaccines or problematic DTaP vaccines-a vaccine that can prevent diphtheria, tetanus and whooping cough-produced by the Changchun company or by Wuhan Institute of Biological Products Co.
The two companies sold more than 650,000 ineffective DTaP vaccines to buyers in three provincial regions, including Shandong province, China's top drug authority said in November, adding that the vaccines do not pose health risks.
The administration has suspended buying and using vaccines from Changchun Changsheng Bio-tech Co to protect the health of international travelers, it said.
The Shandong provincial Center for Disease Control and Prevention said on Wednesday that it had made full preparations following disclosure of the ineffective vaccines, and will give DTaP vaccines again to children who received the substandard vaccines on a voluntary basis.
Children who have had any of the three diseases-diphtheria, tetanus and whooping cough-before 5 years of age can claim compensation from the producers if they have received substandard vaccines, the center said.
Health authorities in Chongqing and Hebei also said they would give vaccinations to any children affected.
In its statement, the WHO reiterated that quality-assured vaccines are critical for disease prevention and urged countries to continue using this cost-effective public health intervention.
- Poll shows rising interest in Chinese culture
- Hero driver dies after saving South Korean tourists
- Gratitude poster honors Xinjiang officer who braved flood to save tourists
- Maritime Day events held in China's Hainan
- Intl symposium discusses major global earthquakes and prediction research
- Rare birds return to Dongting Lake wetlands