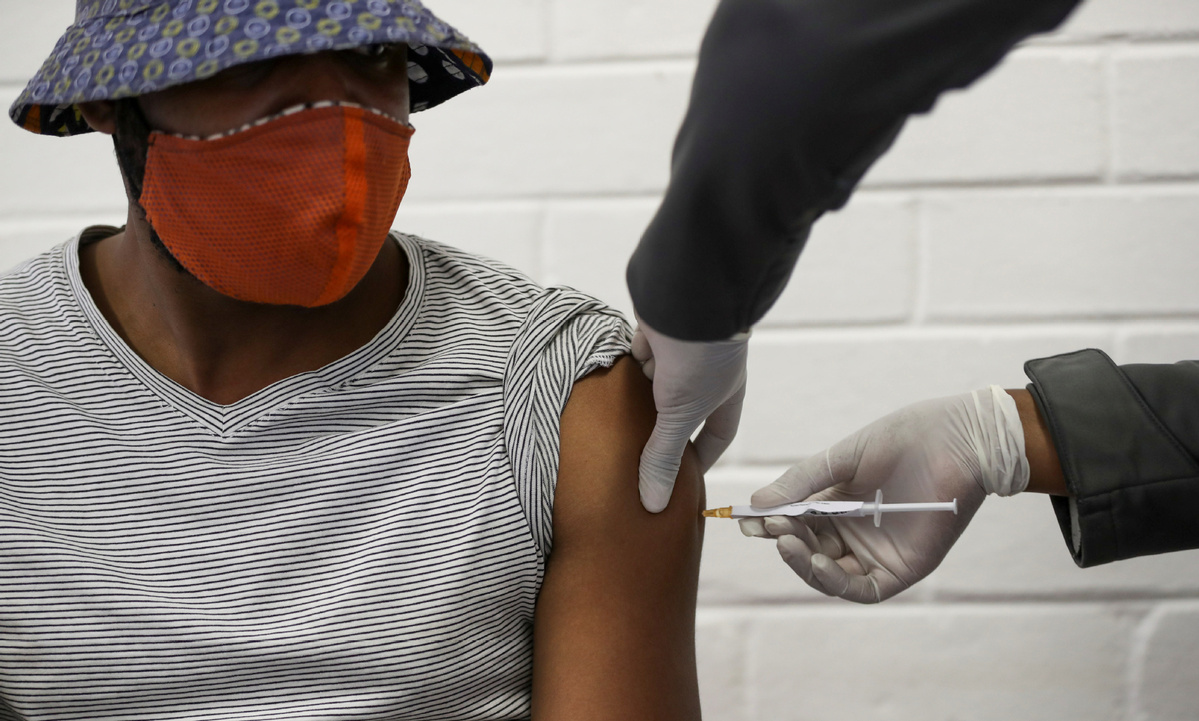
Africans working to speed up vaccine trials
Consortium formed to help complete clinical tests for promising candidates

Consortium formed to help complete clinical tests for promising candidates
The African Union Commission has launched a project to oversee COVID-19 vaccine trials across the continent.
The project, called the Consortium for COVID-19 Vaccine Clinical Trial, is aimed at helping the early completion of more than 10 late-stage vaccine clinical trials on the continent by bringing together global vaccine developers and funders, as well as African organizations that facilitate clinical trials.
According to the AU Commission, the goal is to ensure sufficient data is generated on the safety and efficacy of the most promising vaccine candidates for the African population so they can be confidently rolled out in the continent once the vaccines are approved.
The consortium will focus on dismantling barriers to clinical trials by establishing partnerships with leading vaccine developers to host selected late-stage trial sites.
This is in addition to identifying countries and regions where opportunities to conduct trials are most promising, for example the availability of strong local trial sponsors, good clinical practice investigators, access to epidemiologic data and support from regulatory bodies.
It will also focus on setting up an independent review board to provide guidance, assistance and oversight to clinical trials on matters such as regulation, ethics, and safety.
Another focus will be engaging with African and global media and key opinion leaders on the continent to increase public awareness of the need and importance of hosting well-regulated clinical trials.
This is in addition to providing objective, fact-based scientific guidance for interpreting the results of clinical trial data, and engaging global donors that are interested in investing in scaling-up vaccine distribution in Africa to raise sufficient funds to support the trials.
Encouraging initiative
Moustafa Mijiyawa, chair of the governing board of the Africa Centres for Disease Control and Prevention and Togo's minister of health, said the African initiative is welcomed and encouraging.
"Through this initiative, Africa will be at the forefront as the world seeks to overcome the coronavirus pandemic," he said.
The consortium is the outcome of the virtual conference on Africa's Leadership Role in COVID-19 Vaccine Development and Access held over June 24-25.
The initiative is being implemented as part of the Africa Joint Continental Strategy for COVID-19 Outbreak endorsed by African health ministers on Feb 22, and approved by the Bureau of the Assembly of African Union Heads of State and Government on March 26.
John Nkengasong, the director of Africa CDC, said the consortium will also be a platform that will engage in other discussions being led by GAVI, a vaccine alliance, to negotiate Africa's position on vaccine access and development.
"I want to assure the community that Africa CDC through the consortium will ensure that vaccine trials in Africa are done in the most safe and appropriate manner using established methods," he said.
Nkengasong said Africa CDC will embark on a multi-stakeholder partnerships drive to advance the consortium and other subsidiary initiatives, to ensure broad endorsement and support across Africa, by institutions and the African people.
"Africa must play an active role in securing an effective vaccine against COVID-19. This is about our future and our development," he said.
Nkengasong said there was a lot of misinformation surrounding the ongoing vaccine trials across the globe, and called on the media to publish correct information on vaccine trials.
"A vaccine is one of the most popular tools that have been proved effective and a game changer in pandemics over the history of infectious diseases. It's the only weapon that will allow our lives to return to normal," he said.
Moussa Faki Mahamat, the chairman of the AU Commission, said it was critically important for academics, researchers and the private sector to work together and use all available platforms for the development of the coronavirus vaccine, which would enable Africa to regain momentum for achieving the goals of the continental integration agenda.